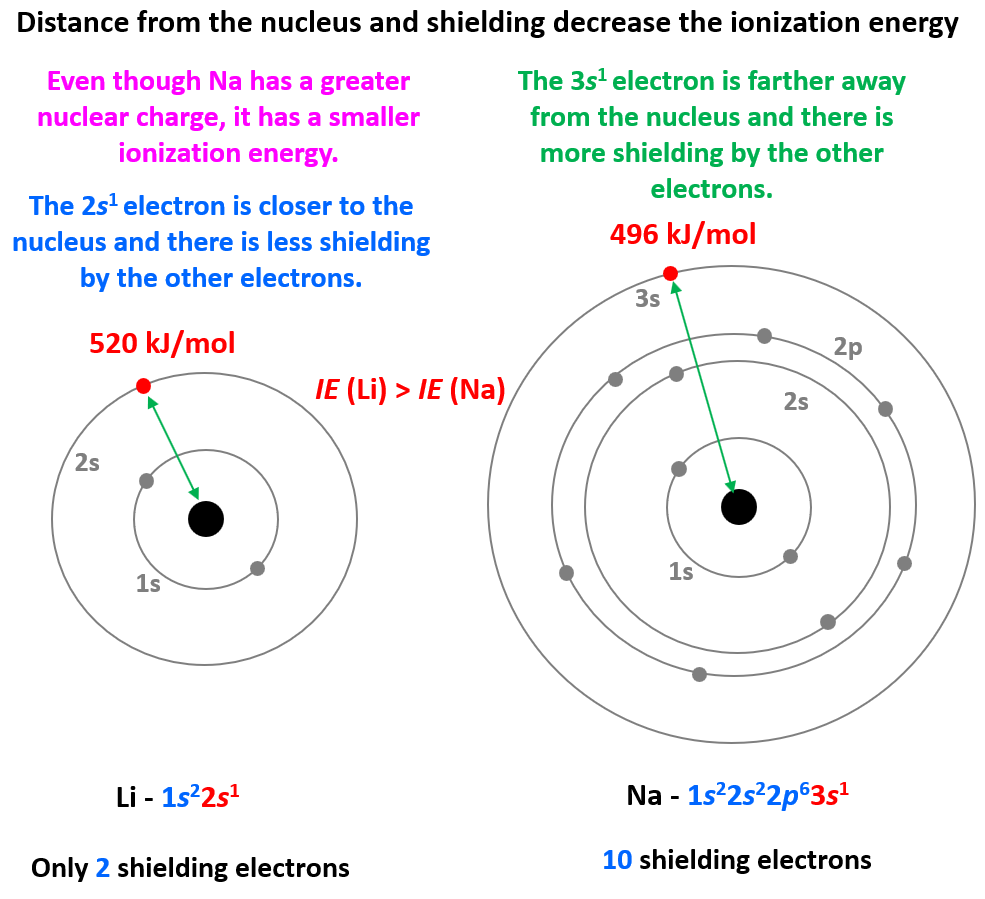
Electron Shielding Down A Group . electron affinity generally decreases down a group of elements because each atom is larger than the atom above it (this is the atomic radius trend,. Along the period from left to right along a. electron shielding refers to the blocking of valence shell electron attraction by the nucleus, due to the presence of. Complete electron shells shield the. shielding increases down a group because the nuclear core is farther removed from the valence electrons. the concept of the shielding effect explains the variation of atomic radius, ionization energy, etc. evaluate how electron shielding contributes to periodic trends such as atomic radius and ionization energy across a period and. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use.
from general.chemistrysteps.com
electron shielding refers to the blocking of valence shell electron attraction by the nucleus, due to the presence of. Complete electron shells shield the. the concept of the shielding effect explains the variation of atomic radius, ionization energy, etc. evaluate how electron shielding contributes to periodic trends such as atomic radius and ionization energy across a period and. electron affinity generally decreases down a group of elements because each atom is larger than the atom above it (this is the atomic radius trend,. shielding increases down a group because the nuclear core is farther removed from the valence electrons. Along the period from left to right along a. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use.
Ionization energy Chemistry Steps
Electron Shielding Down A Group Complete electron shells shield the. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. electron affinity generally decreases down a group of elements because each atom is larger than the atom above it (this is the atomic radius trend,. Along the period from left to right along a. electron shielding refers to the blocking of valence shell electron attraction by the nucleus, due to the presence of. the concept of the shielding effect explains the variation of atomic radius, ionization energy, etc. evaluate how electron shielding contributes to periodic trends such as atomic radius and ionization energy across a period and. Complete electron shells shield the. shielding increases down a group because the nuclear core is farther removed from the valence electrons.
From slideplayer.com
New topic The Periodic Table ppt download Electron Shielding Down A Group evaluate how electron shielding contributes to periodic trends such as atomic radius and ionization energy across a period and. Along the period from left to right along a. shielding increases down a group because the nuclear core is farther removed from the valence electrons. electron shielding refers to the blocking of valence shell electron attraction by the. Electron Shielding Down A Group.
From slidetodoc.com
Trends in the Periodic Table IDENTIFYING THE PATTERNS Electron Shielding Down A Group evaluate how electron shielding contributes to periodic trends such as atomic radius and ionization energy across a period and. the concept of the shielding effect explains the variation of atomic radius, ionization energy, etc. shielding increases down a group because the nuclear core is farther removed from the valence electrons. electron affinity generally decreases down a. Electron Shielding Down A Group.
From slideplayer.com
Periodic Trends. ppt download Electron Shielding Down A Group evaluate how electron shielding contributes to periodic trends such as atomic radius and ionization energy across a period and. Along the period from left to right along a. Complete electron shells shield the. electron shielding refers to the blocking of valence shell electron attraction by the nucleus, due to the presence of. the concept of electron shielding,. Electron Shielding Down A Group.
From www.chem.fsu.edu
Electron Configurations Electron Shielding Down A Group evaluate how electron shielding contributes to periodic trends such as atomic radius and ionization energy across a period and. Along the period from left to right along a. shielding increases down a group because the nuclear core is farther removed from the valence electrons. Complete electron shells shield the. the concept of electron shielding, in which intervening. Electron Shielding Down A Group.
From slideplayer.com
Section 3 Periodic Trends ppt download Electron Shielding Down A Group the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. the concept of the shielding effect explains the variation of atomic radius, ionization energy, etc. electron shielding refers to the blocking of valence shell electron attraction by the nucleus, due to the presence of.. Electron Shielding Down A Group.
From slideplayer.com
Chemsheets AS006 (Electron arrangement) ppt download Electron Shielding Down A Group evaluate how electron shielding contributes to periodic trends such as atomic radius and ionization energy across a period and. electron affinity generally decreases down a group of elements because each atom is larger than the atom above it (this is the atomic radius trend,. Along the period from left to right along a. Complete electron shells shield the.. Electron Shielding Down A Group.
From www.slideserve.com
PPT Chapter 6 The Periodic Table PowerPoint Presentation, free Electron Shielding Down A Group electron shielding refers to the blocking of valence shell electron attraction by the nucleus, due to the presence of. shielding increases down a group because the nuclear core is farther removed from the valence electrons. electron affinity generally decreases down a group of elements because each atom is larger than the atom above it (this is the. Electron Shielding Down A Group.
From www.slideserve.com
PPT Periodic Table Chapter PowerPoint Presentation, free download Electron Shielding Down A Group the concept of the shielding effect explains the variation of atomic radius, ionization energy, etc. electron shielding refers to the blocking of valence shell electron attraction by the nucleus, due to the presence of. Complete electron shells shield the. evaluate how electron shielding contributes to periodic trends such as atomic radius and ionization energy across a period. Electron Shielding Down A Group.
From www.youtube.com
S3.1.3 Electron shielding and effective nuclear charge YouTube Electron Shielding Down A Group the concept of the shielding effect explains the variation of atomic radius, ionization energy, etc. electron affinity generally decreases down a group of elements because each atom is larger than the atom above it (this is the atomic radius trend,. electron shielding refers to the blocking of valence shell electron attraction by the nucleus, due to the. Electron Shielding Down A Group.
From slideplayer.com
Elemental Properties and Patterns ppt download Electron Shielding Down A Group the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. Complete electron shells shield the. electron affinity generally decreases down a group of elements because each atom is larger than the atom above it (this is the atomic radius trend,. shielding increases down a. Electron Shielding Down A Group.
From slidetodoc.com
The Periodic Table The how and why History Electron Shielding Down A Group Complete electron shells shield the. shielding increases down a group because the nuclear core is farther removed from the valence electrons. Along the period from left to right along a. the concept of the shielding effect explains the variation of atomic radius, ionization energy, etc. electron shielding refers to the blocking of valence shell electron attraction by. Electron Shielding Down A Group.
From www.slideserve.com
PPT Unit 5 The Periodic Table PowerPoint Presentation, free download Electron Shielding Down A Group electron affinity generally decreases down a group of elements because each atom is larger than the atom above it (this is the atomic radius trend,. shielding increases down a group because the nuclear core is farther removed from the valence electrons. the concept of the shielding effect explains the variation of atomic radius, ionization energy, etc. . Electron Shielding Down A Group.
From www.showme.com
Electron shielding Science, Chemistry, Electrons, electron shielding Electron Shielding Down A Group evaluate how electron shielding contributes to periodic trends such as atomic radius and ionization energy across a period and. Complete electron shells shield the. electron shielding refers to the blocking of valence shell electron attraction by the nucleus, due to the presence of. the concept of electron shielding, in which intervening electrons act to reduce the positive. Electron Shielding Down A Group.
From www.youtube.com
Electron shielding YouTube Electron Shielding Down A Group evaluate how electron shielding contributes to periodic trends such as atomic radius and ionization energy across a period and. shielding increases down a group because the nuclear core is farther removed from the valence electrons. Complete electron shells shield the. the concept of the shielding effect explains the variation of atomic radius, ionization energy, etc. electron. Electron Shielding Down A Group.
From slideplayer.com
Coulomb’s Law PERIODIC TRENDS Nuclear Charge Electron Shielding ppt Electron Shielding Down A Group shielding increases down a group because the nuclear core is farther removed from the valence electrons. the concept of the shielding effect explains the variation of atomic radius, ionization energy, etc. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. Complete electron shells. Electron Shielding Down A Group.
From slideplayer.com
Periodic Trends OBJECTIVES ppt download Electron Shielding Down A Group the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. Along the period from left to right along a. evaluate how electron shielding contributes to periodic trends such as atomic radius and ionization energy across a period and. shielding increases down a group because. Electron Shielding Down A Group.
From slideplayer.com
Metals. Metals Scientists Families Vocab Trends. ppt download Electron Shielding Down A Group shielding increases down a group because the nuclear core is farther removed from the valence electrons. the concept of the shielding effect explains the variation of atomic radius, ionization energy, etc. evaluate how electron shielding contributes to periodic trends such as atomic radius and ionization energy across a period and. electron affinity generally decreases down a. Electron Shielding Down A Group.
From general.chemistrysteps.com
Ionization energy Chemistry Steps Electron Shielding Down A Group electron shielding refers to the blocking of valence shell electron attraction by the nucleus, due to the presence of. shielding increases down a group because the nuclear core is farther removed from the valence electrons. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the. Electron Shielding Down A Group.